Purpose: To determine if the water sample meets the Canadian Drinking Water Guideline for pH by determining its level of pH. Testing will be done on Local community treated water. There will be a pH 7 buffer solution included for quality control purposes.
There is a 7.0-10.5 range in the Canadian Drinking Water Guideline for pH in drinking water; you will test and compare your result to see if it meets these guidelines. For demonstration purposes get two other samples like Coke and bleach to show the pH levels of these compared to the drinking water.
Materials:
1 – 10 mL vial containing 4 pH test strips
1 – 5 mL vial containing pH 7 buffer
1 - pH scale card
3 – 10 mL disposable beakers
2 - example samples
Method:
Label the three beakers Local, and what the other two samples are (such as Coke and Bleach) (do not include the buffer; this can be tested in the tube).
Fill the beakers with their respective samples.
Place the pH strip into the beakers/ vial.
Leave for 2 minutes.
Remove the pH strip and lay it across the beaker, coloured side up. Wait 30 seconds.
Determine the pH of the strip by comparing it to the pH scale card.
Record your results.
Record Your Results
Results:
Sample water with a pH between 7.0 and 10.5 meets the Canadian Drinking Water Guideline for pH. The buffer should give a result very close to 7.
Visit the Safe Drinking Water Foundation Website www.safewater.org to learn more about issues affecting safe drinking water.
pH
What is pH and why do we test our water for it?
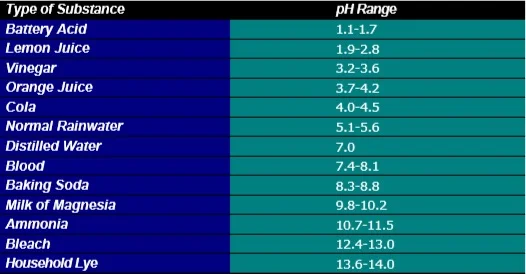
pH is an index of the amount of hydrogen ions (H + ) are in a substance. The pH scale runs from 0-14, with 7.0 being neutral. Substances with a pH higher than 7.0 (7.1-14.0) are considered alkaline or basic. Substances with a pH less than 7.0 (0 - 6.9) are considered acidic. We consume many different foods and beverages with a large range of pH. For example, citrus fruits like oranges, lemons and limes are quite acidic (pH = 2.0 - 4.0). On the other hand, egg whites are a little basic, with a pH of 8.0. The ideal pH range for water is between 7.2 and 7.6. This means that the water is slightly basic. By maintaining the proper alkalinity of water, the pH will stay around the ideal levels. However, if the alkalinity gets too low, the pH can start to deviate and can begin to cause water quality problems.
What happens if the pH of my water is too low or too high?
There are no health risks associated with consuming water that is slightly acidic or basic. After all, we can eat lemons, drink soft drinks, and eat eggs. However, when water has a pH that is too low, it will lead to corrosion and pitting of pipes in plumbing and distribution systems. This can lead to health problems if metal particles are leached into the water supply from the corroded pipes. The water also has a slightly bitter and metallic taste that some may find objectionable. If the pH of your water is too high, it will have a taste similar to baking soda and will have a slippery feel to it. It will also begin to leave scale deposits on plumbing and fixtures, which will decrease the efficiency of the plumbing systems.
pH of several different substances
Source: http://bear_creek.tripod.com/water.htm
How do I increase or decrease the pH of my water?
Acidic water can be corrected using one of the following two methods:
1. Neutralizing filters increase the pH by passing water through a filter bed of Calcium Carbonate (CaCO3). This neutralizes the acid and increases the pH.
2. Soda Ash (Sodium Carbonate) solution is fed through a tube into the pumping intake and is automatically injected whenever the water pump is running.
NOTE: Both Sodium and Calcium Carbonate are the most common compounds used to increase pH in drinking water.
Basic water can be corrected by either adding a specific volume of Muriatic acid (hydrochloric acid) or a commercially prepared chemical designed to decrease the pH. It is always best to check with water treatment experts when deciding on t he products and the volumes to use when adjusting pH.