TDS AND pH FACT SHEET
What are TDS?
TDS stands for total dissolved solids, and represents the total concentration of dissolved substances in water. TDS is made up of inorganic salts, as well as a small amount of organic matter. Common inorganic salts that can be found in water include calcium, magnesium, potassium and sodium, which are all cations, and carbonates, nitrates, bicarbonates, chlorides and sulfates, which are all anions. Cations are positively charged ions and anions are negatively charged ions.
How do These Solids End Up Dissolved in Water?
These minerals can originate from a number of sources, both natural and as a result of human activities. Mineral springs contain water with high levels of dissolved solids, because the water has flowed through a region where the rocks have a high salt content. The water in the Prairie provinces tends to have high levels of dissolved solids, because of high amounts of calcium and magnesium in the ground.
These minerals can also come from human activities. Agricultural and urban runoff can carry excess minerals into water sources, as can wastewater discharges, industrial wastewater and salt that is used to de-ice roads.
What Happens to the Water When the TDS Level is High?
Alone, a high concentration of dissolved solids is usually not a health hazard. In fact, many people buy mineral water, which has naturally elevated levels of dissolved solids. The United States Environmental Protection Agency (EPA), which is responsible for drinking water regulations in the United States, includes TDS as a secondary standard, meaning that it is a voluntary guideline in the United States. While the United States set legal standards for many harmful substances, TDS, along with other contaminants that cause aesthetic, cosmetic and technical effects, has only a guideline.
Most people think of TDS as being an aesthetic factor. In a study by the World Health Organization, a panel of tasters came to the following conclusions about the preferable level of TDS in water:
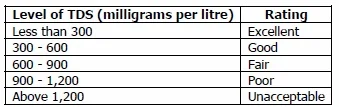
Taste of Water with Different TDS Concentrations;
https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/tds.pdf?sfvrsn=3e6d651e_4
However, a very low concentration of TDS has been found to give water a flat taste, which is undesirable to many people.
Increased concentrations of dissolved solids can also have technical effects. Dissolved solids can produce hard water, which leaves deposits and films on fixtures, and on the insides of hot water pipes and boilers. Soaps and detergents do not produce as much lather with hard water as with soft water. As well, high amounts of dissolved solids can stain household fixtures, corrode pipes, and have a metallic taste. Hard water causes water filters to wear out sooner, because of the amount of minerals in the water. The picture below was taken near the Mammoth Hot Springs, in Yellowstone National Park, and shows the effect that water with high concentrations of minerals can have on the landscape. The same minerals that are deposited on these rocks can cause problems when they build up in pipes and fixtures.
Mineral Deposition from Minerals in Water at Mammoth Hot Springs
However, while TDS itself may be only an aesthetic and technical factor, a high concentration of TDS is an indicator that harmful contaminants, such as iron, manganese, sulfate, bromide and arsenic, can also be present in the water. This is especially true when the excessive dissolved solids are added to the water as human pollution, through runoff and wastewater discharges.
What are the Guidelines for TDS?
In Canada, substances that are considered to be dangerous in high amounts are listed as Maximum Acceptable Concentrations (MACs) in the Canadian Guidelines for Drinking Water Quality. However, substances that are not considered dangerous at their MAC, such as TDS, are given an aesthetic objective in the Guidelines. The Canadian guideline for TDS is less than 500 milligrams per litre (which is the same as 500 parts per million). However, since the Canadian guidelines are not enforceable, each province is free to choose whether or not they will follow the guidelines. Saskatchewan has water that naturally contains high concentrations of TDS, so the province has chosen to not follow the Canadian guideline of 500 parts per million, and to implement its own guideline of 1,500 parts per million.
In the United States, substances that are health-based have Maximum Contaminant Levels (MCLs), and are enforceable by law. However, TDS, and other substances that are considered aesthetic, are given Secondary Maximum Contaminant Levels (SMCLs), but are not enforced, because they do not pose as great a health risk as the primary contaminants do. The United States guideline for TDS is also 500 parts per million.
How Can Water Treatment Facilities Remove TDS?
Water treatment facilities can use reverse osmosis to remove the dissolved solids in the water that are responsible for elevated TDS levels. Reverse osmosis removes virtually all dissolved substances, including many harmful minerals, such as salt and lead. It also removes healthy minerals, such as calcium and magnesium, and ideally such water should be filtered through a magnesium and calcium mineral bed to add the minerals to the water. The mineral bed also increases the pH and decreases the corrosive potential of the water. For more information about reverse osmosis, see the Ultrafiltration, Nanofiltration and Reverse Osmosis fact sheet.
What is pH?
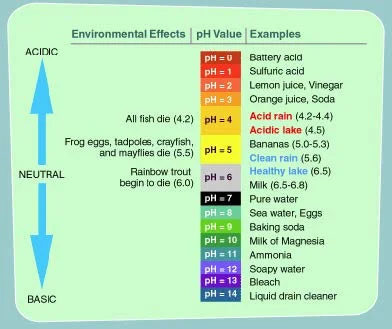
The pH value of a water source is a measure of its acidity or alkalinity. The pH level is a measurement of the activity of the hydrogen atom, because the hydrogen activity is a good representation of the acidity or alkalinity of the water. The pH scale, as shown below, ranges from 0 to 14, with 7.0 being neutral. Water with a low pH is said to be acidic, and water with a high pH is basic, or alkaline. Pure water would have a pH of 7.0, but water sources and precipitation tends to be slightly acidic, due to contaminants that are in the water.
pH Scale
The pH scale is logarithmic, which means that each step on the pH scale represents a ten-fold change in acidity. For example, a water body with a pH of 5.0 is ten times more acidic than water with a pH of 6.0. And water with a pH of 4.0 is 100 times more acidic than water with a pH of 6.0.
How Does the pH of a Water Source Change?
Surface water typically has a pH value between 6.5 and 8.5 and groundwater tends to have a pH between 6.0 and 8.5. The pH of a water source can vary naturally. Some types of rock and soil, such as limestone, can neutralize acid more effectively than other types of rock and soil, such as granite. Or, when there are a large number of plants growing in a lake or river, they release carbon dioxide when they die and decompose. When the carbon dioxide mixes with the water, a weak carbonic acid is formed; this can then cause the pH of the water body to decrease.
A number of human activities have a harmful effect on the pH of nearby water sources. When sulfur dioxide and nitrogen oxides are emitted, through industrial operations and vehicles, acid rain can be produced. For more information about acid rain, see the Acid Rain fact sheet.
Chemical pollution, from industrial operations, individuals and communities, can cause a water body to become acidic. These chemicals can enter the water through illegal discharges or after inadequate wastewater treatment. For more information about chemical pollution, including ways in which you can minimize pollution, see the Water Pollution fact sheet.
What Happens When the pH of the Water Changes?
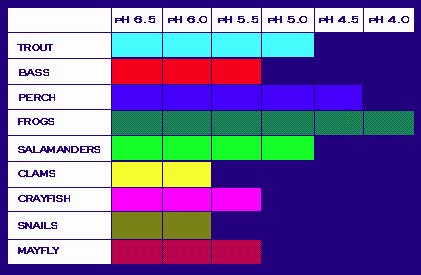
A change in the pH of water can have a number of consequences. In the environment, many plants and animals are harmed, or even killed, as a result of acidification. Many varieties of fish and aquatic life are extremely sensitive to changes in water temperature and composition. The below diagram illustrates the pH that is required for a number of aquatic species. Notice that when the pH is around 6.0 to 7.0 (which is natural for many lakes and streams), the biodiversity within the ecosystem is wide. As the pH decreases and the acidity increases, fewer and fewer organisms can survive.
Required pH Level for the Survival of Common Species of Fish
Acidic water is synergistic, which means that a combination of a low pH and an increased concentration of certain substances is far more harmful than the sum of the parts. For example, aluminium, lead and mercury are potentially dangerous substances, but when the pH of the water source is already low, these substances can have extremely detrimental consequences for aquatic life.
Acidic water can also cause problems for human consumption. While slightly acidic water is not dangerous, on its own, it can be quite dangerous when combined with other compounds. Water with a pH that is less than 6.5 can leach metal ions, including iron, manganese, copper, lead and zinc from plumbing fixtures and pipes. This, in return, can be quite dangerous. On the other end of the pH scale, water that has a pH greater than 8.0 can be difficult to disinfect. The World Health Organization recommends that the pH of the water be less than 8.0, because basic water does not allow for effective chlorination.
What are the Guidelines for pH?
Like TDS, pH is given an aesthetic objective in Canada. The Canadian Guidelines for Drinking Water Quality suggest that the pH of drinking water should be between 7.0 and 10.5. The Saskatchewan Drinking Water Standards and Objectives recommend that the pH of drinking water be between 6.5 and 9.0.
In the United States, pH is, like TDS, a secondary standard; the Secondary Maximum Contaminant Level for pH is between 6.5 and 8.5. According to the EPA, the noticeable effects of a pH that is less than 6.5 include a bitter, metallic taste and corrosion. The noticeable effects of a pH above 8.5 include a slippery feeling, soda-like taste and deposits.
How do Water Treatment Facilities Change the pH of Water?
There are several methods that can increase the pH of water, before disinfection. The pH is commonly increased using sodium carbonate and sodium hydroxide, but a better way of dealing with low pH is to use calcium and magnesium carbonate, which not only will increase pH levels, but will also make the water less corrosive and both calcium and magnesium are of health benefits as opposed to sodium.
Why is it Important to Monitor TDS and pH?
It is important to monitor the TDS level and the pH of drinking water for several reasons. When a water source has a high level of TDS or a low pH, it is likely that there are other harmful contaminants in the water. Both TDS and pH are also easy to measure and if something is happening to a water, such as pollution, chances are both TDS and pH levels will change so keeping track of those changes can act as an early warning signal that something is happening to the water. For these reasons, it is important to monitor the TDS and pH levels, so that if they change, action can be taken immediately.
For more information about TDS and pH, including the ways in which you can use these tests on your drinking water, see the Operation Water Pollution program.
The Safe Drinking Water Foundation has educational programs that can supplement the information found in this fact sheet. Operation Water Drop looks at the chemical contaminants that are found in water; it is designed for a science class. Operation Water Flow looks at how water is used, where it comes from and how much it costs; it has lessons that are designed for Social Studies, Math, Biology, Chemistry and Science classes. Operation Water Spirit presents a First Nations perspective of water and the surrounding issues; it is designed for Native Studies or Social Studies classes. Operation Water Health looks at common health issues surrounding drinking water in Canada and around the world and is designed for a Health, Science and Social Studies collaboration. Operation Water Pollution focuses on how water pollution occurs and how it is cleaned up and has been designed for a Science and Social Studies collaboration. To access more information on these and other educational activities, as well as additional fact sheets, visit the Safe Drinking Water Foundation website at www.safewater.org.
Did you find this information useful? Please help us to send digital TDS and digital pH meters which are guaranteed to work for at least two years to schools! Please chip in $5 or donate $20 or more and receive an Official Donation Receipt for Income Tax Purposes - or donate $170 to provide an Operation Water Pollution kit for a school.
Resources:
Government of Canada. August 2016. Canadian Drinking Water Guidelines.
https://www.canada.ca/en/health-canada/services/environmental-workplace-health/water-quality/drinking-water/canadian-drinking-water-guidelines.html
Government of Saskatchewan. n.d. Saskatchewan’s Drinking Water Quality Standards and Objectives (Summarized).
https://publications.saskatchewan.ca/api/v1/products/112863/formats/126899/download
Water Research Center. n.d. The pH of Water.
http://www.water-research.net/index.php/ph
Water Research Center. n.d. Total Dissolved Solids (TDS).
http://www.water-research.net/index.php/water-treatment/tools/total-dissolved-solids
Water Research Center. n.d. pH in the Environment.
http://www.water-research.net/index.php/ph-in-the-environment
World Health Organization. 2011. Guidelines for Drinking-water Quality.
https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf
World Health Organization. 1996. Total dissolved solids in Drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality.
https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/tds.pdf?sfvrsn=3e6d651e_4