CLEANING UP AFTER POLLUTION FACT SHEET
“It wasn’t the Exxon Valdez captain’s driving that caused the Alaskan oil spill. It was yours.”
- Greenpeace advertisement, New York Times, February 25, 1990.
It is very difficult to reverse the effects of water pollution. Natural processes that cleanse the water can take years, decades, or even centuries, and even with costly technological processes, it can take years to remove all of the harmful substances from the water. There are two aspects of the contamination that must be removed. First, and most importantly, the source of the water pollution must be removed, so that additional water contamination does not occur.
How Can the Pollution Source be Removed?
Removing pollution sources occurs in a number of ways, depending on the source (and whether the pollution originates from a point source or a non-point source), and the type of pollution. Removing the source can be as easy as digging up a leaking oil tank or as difficult as legislating controls on a toxic substance. Unfortunately, industrial and agricultural pollution practices are generally not minimized until government regulations are developed to set limits on air and effluent emissions. The regulations typically determine the amount of pollutants that can be emitted, as well as how and where wastes may be disposed of.
In the United States, the Clean Water Act sets standards for water quality, based on three key components. First, an antidegradation statement is made, which states the conditions under which water quality is allowed to be lowered. Secondly, the stream use is classified as domestic water supply, industrial water supply, fish and aquatic life, recreation, irrigation, livestock watering, wildlife or navigation. The third component is the water quality criteria, or the degree of water quality that is required for its designated use. This act allows the quality of streams intended for industrial purposes to be degraded more than a stream intended to provide domestic drinking water, for example.
In some countries, if companies exceed pollution limits, they can be fined. Various countries, including some Canadian provinces and the United States, allow companies to buy and sell pollution credits. Pollution trading is much more common in the United States, but several provinces in Canada have developed pollution trading markets. For example, according to the Edmonton Journal, in July 2007, the province of Alberta was planning to launch a greenhouse gas emissions trading market. In 2002, the Ontario government introduced a smog trading program, in the hopes that it would reduce air pollution in the province.
In general, pollution trading programs mean that companies that cause very little pollution can sell credit to companies that exceed their pollution limit. In the United States, trading must meet requirements of the Clean Water Act, which regulates types and amounts of pollution, and trading must also occur within the same watershed. The Canadian policies tend to be less well developed than the American policies. For example, the Ontario policy proposed to allow transboundary trading between the United States and Canada; the Environmental Protection Agency, who developed the trading policies in the United States, does not allow companies to trade with companies in other countries.
Cities can reduce water pollution by upgrading their wastewater treatment facilities. Most urban centres have wastewater facilities with secondary treatment processes, but installing a tertiary treatment process can remove phosphorus, which is responsible for excess algae growth. For more information about various methods of wastewater treatment, see the fact sheet titled Wastewater Treatment.
The most beneficial action that individuals can take is to reduce pollution within their own lives. Using less and proper disposal of harmful substances can go a long way in removing or minimizing sources of water pollution. For more information about water pollution, in general, including pollution-reducing actions, see the Water Pollution fact sheet.
Pollution Comes from so Many Directions and Sources; is it Really Possible to Remove Enough of the Sources that Water Quality Can Improve?
In the 1970s, there was widespread concern that Lake Erie was dying. There was excess algae growth and oxygen levels were low, which was killing fish and aquatic life. The lake was green, slimy and smelly, and the only life in the lake was the algae, which had overtaken all other aquatic plants and animals. The Cuyahoga River, which flows into Lake Erie near Cleveland, caught fire because of the oil on its surface. The quality of the other four lakes was not much better than Lake Erie. For more information about the Great Lakes, see the Great Lakes fact sheet and the social studies lesson plan in Operation Water Flow called ”A Case Study on Water Pollution in Canada".
In 1972, the Great Lakes Water Quality Agreement was signed between Canada and the United States, in an effort to reverse the harmful effects that pollution was having on the Great Lakes. Pierre Trudeau stated that the signing of the Agreement marked “our recognition of the fragility of our planet and the delicacy of the biosphere on which all life is dependent. The Agreement deals with the most vital of all issues – the process of life itself… [I]t promises to restore to a wholesome condition an immense area which, through greed and indifference, has been permitted to deteriorate disgracefully”. But removing the sources of pollution around the Great Lakes would not be an easy task that could be quickly completed. In 1970, the Great Lakes region was home to approximately 30 million people, which is the equivalent to the entire population of Canada! The region is also strongly influenced by intense industrial and agricultural operations.
In 1978, the Agreement called to eliminate discharges of persistent toxic substances, such as PCBs. In 1983, phosphorus discharge limits were set, and in 1987, the Agreement expanded to include airborne pollutants. Also in 1987, Remedial Action Plans were developed for 42 local areas of concern (another was added later). Attention was focused on reducing runoff, sediment pollution, airborne toxic substances and contaminated groundwater. Some cities were given grants to upgrade the quality of their wastewater treatment facilities and standards were set for waste emissions from commercial ships. Chicago was the first city to ban phosphates in detergents. In 1974, a recreation area comprising about 135 square kilometers along 35 kilometres of the Cuyahoga River was created.
Factories Near The Great Lakes
Great efforts were required, and are still required, to reduce the impact of pollution that enters the Great Lakes. The first area of concern to be restored was Collingwood Harbour, Ontario, on Lake Huron; it was not completed until 1994. While there are still many areas of concern, the progress that has been made demonstrates that a combination of regulations and concerned citizens, organizations and industries can control pollution and minimize contamination of nearby water sources. For more information about the Great Lakes, including the state that they are currently in, see the Great Lakes fact sheet.
Why Does it Take so Long to Remove the Contaminants from the Water?
Most contaminants are water soluble, meaning that they dissolve in water. Water sources can dilute contaminants to a concentration that may not be dangerous. However, after enough pollution, the capacity of the lake, river or stream can be exceeded. The retention time, or residence time, of a water body is the time that it takes for a substance that is introduced to the water to flow out again. The retention time of a lake can vary, depending on the volume of the lake, the depth of the lake, and the number of rivers flowing in and out of the lake. While some lakes have retention times of only several months, there are others that retain substances for several thousand years. The following chart summarizes the retention times of several lakes.
Retention Time of Water in Various Water Bodies;
Environment Canada and http://en.wikipedia.org/wiki/Lake_retention_time
Even after harmful substances leave a lake and pass on to groundwater or a river, many of the contaminants still remain in the water. This is because many common household products contain chemicals that take many years to break down. The following table lists the time required to decompose in a landfill for a number of items.
Degradation Time in Landfills For Various Household Items
The following table lists the time that is required for some common items to break down in water.
Degradation Time in Water For Various Household Items
It is important to realize that all of these times are only estimates, and the actual time to decompose can vary, according to how compact the waste is, the chemical composition of the item, and the temperature, sunlight and precipitation that the waste receives. Some glass, plastics and metals can take centuries or millennia to decompose. The important information to take away from this chart is that many common household items will be around for generations to come.
How Can Water Sources that are Already Contaminated be Cleaned?
The second aspect of water pollution involves cleaning the waters that are already contaminated. It is estimated that it would cost up to one trillion dollars to clean up existing environmental contamination in the United States. There are a number of ways in which this can be accomplished, the most effective being the protection of water sources against future contamination, while allowing natural biological, chemical and physical processes to break down existing contaminants. However, if the water source is used to provide drinking water, additional treatment may be necessary, to improve the water quality in a shorter period of time.
Various factors determine the appropriate type of treatment, including the water source (whether it is groundwater or surface water, for example), the volume and depth of the water, and the type and amount of chemical in the water.
For information about how oil spills are cleaned up, see the fact sheet titled Oil Spills. Contaminated sediment, at the bottom of a lake or riverbed, is especially difficult to remove. It can be removed by dredging, which is the process of removing the contaminated sediment and disposing of it in a safer location. During the restoration of Lake Michigan, contaminated sediment had to be dredged from the bottom of Green Bay. Over many years, a furniture manufacturing plant had discarded paint sludge in Green Bay, and a crust that was almost one metre thick had built up! The paint contained dangerous concentrations of lead, metals and organic compounds. To remove the contaminated sludge, a rock dyke was built to prevent paint chunks from washing further into the water and 13,000 kilograms of waste was removed from the water, treated, and properly disposed of elsewhere. In cases where dredging is not an option, a cover can be placed over the contaminated sediment, to prevent it from coming into contact with the water.
Pump and Treat
In this conventional type of groundwater treatment, pumps are used to bring polluted water to the surface, where it can be more easily treated. Pumping the water out of the ground is a process that works in situations that are difficult to treat, but it can be costly and time-consuming, as the water must be removed from the source and treated. Sometimes, treatment can be applied at the site of contamination, and at other times, the water must be transported, treated and then returned to the source or another location.
This process typically takes between five and ten years to remediate a water source, but it is not unusual for the process to go on for decades. The following three treatment processes, air stripping, activated carbon filtering and bioremediation, are often used as a part of the pump and treat method of remediating a region. Phytoremediation and chemical oxidation are described later, but these two processes do not require the groundwater to be pumped up through a well in order to remove the contaminants.
Air Stripping
Air stripping is a method that uses air to remove contaminants from water. This process can effectively remove chemicals that evaporate easily, including fuels and solvents. Contaminated water is pumped through a large chamber, where it is sprayed over packing material. The packing material allows the water to slowly trickle to the bottom of the tank. At the same time, a fan blows air upwards, which causes the chemicals to evaporate out of the water. The chemicals are collected at the top of the tank, and treated, so that they cannot cause further pollution.
Air Stripping Process
Air strippers can be brought to the site of contamination, which reduces the transportation costs. The rate at which air stripping can remediate a groundwater source varies, but it usually takes many years to clean up a site.
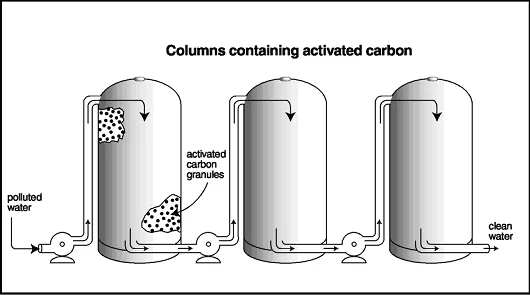
Activated Carbon Filtration
Another process that is commonly used to remove pollutants from water is filtration through activated carbon filters. This method can remove fuel, PCBs, dioxins and radioactive wastes. The polluted water is sent through columns of activated carbon; the chemicals stick, or sorb, to the surface and within the pores of the granules, and the clean water flows through. Many people are familiar with activated carbon filters, as these comprise most tap water filters and fish tank filters.
Activated Carbon Filtration Process
When the filters become fouled, or blocked with contaminants, they must be cleaned or replaced. One disadvantage of using activated carbon filtration is that it does not break down the contaminants, such as some of the other methods, such as bioremediation and phytoremediation, do. To clean an activated carbon filter, the carbon is heated and air is pumped through the columns to loosen the contaminants from the carbon; the contaminants are then disposed of in landfills or destroyed with other methods.
Activated carbon filters are generally used in addition to another process, because excessively polluted water can foul the filters quite quickly. The time required to clean up a groundwater source with this method ranges from a few days to years, depending on the rate and success of other treatment methods. These processes are extremely expensive, with the prevention of the pollution in the first place being hundreds to thousands of times less expensive.
Bioremediation
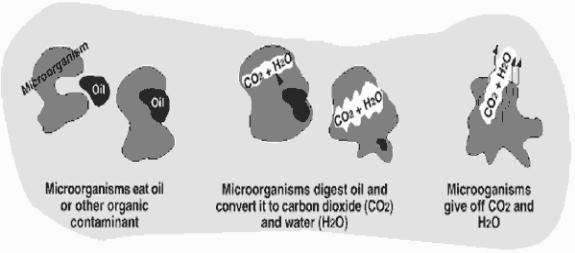
Bioremediation is a process that uses microorganisms that are naturally found in the soil, to digest contaminants in soil and water, including the chemicals that are found in gas and oil spills. As shown in the diagram below, the microorganisms digest the chemicals, and convert them to water and harmless gases, such as carbon dioxide.
Using Micro-organisms To Clean Water
When this process occurs in nature, it is called natural attenuation, and generally occurs at a slow rate. The rate of digestion can be increased with the addition of air and nutrients, which allow the microorganisms to grow and multiply. However, mixing with air can cause chemicals to evaporate before they can be digested, so mixing is often done in an enclosed tank, so that chemicals cannot escape. The water is pumped up through a well and enters a holding tank. Once in the tank, nutrients are added and the water is aerated, to provide optimal conditions for the microorganisms. After enough of the chemicals are removed, the water is pumped back into the aquifer.
Bioremediation can take anywhere from a few months to a few years to remove contaminants, but it is an effective and natural treatment process that does not require the use of chemicals or disinfectants. It is less intrusive than many other forms of treatment and is also fairly inexpensive; in fact, natural attenuation is free!
Bioremediation is a process that is increasingly being used in municipal wastewater treatment plants. For more information about using bioremediation to treat domestic wastewater, see the Wastewater Treatment fact sheet. A process similar to bioremediation can also be used to treat drinking water, especially water that is difficult to treat with any other method.
Phytoremediation
Phytoremediation is a process that uses the roots of plants and trees to remove pollutants, such as metals, pesticides and oil, from soil and water. Trees can remove contaminants that are in deep aquifers, because the roots reach much further than those of small plants. When the roots of plants take in water and nutrients, they also take in the chemicals and store them in their roots, stems and leaves. The plants can then convert the chemicals into gases that are released into the air as the plant transpires, or breathes. Or, the chemicals can stick, or sorb, to the plant roots; in this case, the chemicals are removed from the water or soil only when the plant is removed. A further benefit of phytoremediation is that plants and trees help to prevent further contamination by minimizing runoff and erosion. There is concern, however, about the impact that the plants could have on the ecosystem, as animals feed on plants containing concentrated amounts of toxic chemicals.
Phytoremediation often takes years to restore the water or soil to a high quality, but like bioremediation, it is an effective and natural process and does not rely on chemicals to treat the water, and is relatively inexpensive. For example, to clean one acre of sandy loam soil at a depth of 50 centimetres with phytoremediation would cost between $60,000 and $100,000, while conventional removal and disposal of the same soil would cost around $400,000. Phytoremediation is a process that is similar to the natural process that occurs in wetlands.
Chemical Oxidation
Chemcial oxidation is a process that uses oxidants to convert harmful chemicals, such as fuels, solvents and pesticides, into less harmful chemicals, such as water and carbon dioxide. The most commonly used oxidants are hydrogen peroxide and potassium permanganate, which are liquids. Ozone, which is a gas, can also be used, but it is more difficult to contain than liquids. To apply chemical oxidation to a groundwater source, wells are drilled and the oxidant is pumped in. Often, two wells are dug, so that the water can be circulated, as is shown in the diagram to the right. This ensures that the oxidant is able to be evenly mixed in the water, and remove the majority of the contaminants.
Using Chemical Oxidation to Remove Contaminants From Groundwater
When the oxidant is added, heat is produced; in fact, enough heat is produced to boil water.
This causes the chemicals to evaporate from the water and travel upwards through the soil. The contaminants are captured above ground, where they can be safely treated and disposed of.
Chemical oxidation is an expensive treatment process, but in comparison to bioremediation and phytoremediation, it is a fast process; it generally takes between several months and one year to finish removing the contaminants from a polluted area with oxidation.
So What Should I Get Out of Reading this Fact Sheet?
The most important thing to take away from this fact sheet is that it is always easier and less
expensive to remove the sources of pollution than to continue treating contaminated water. To remediate an area that has been polluted requires efforts on the part of individuals, organizations, industries and governments, who are committed to removing the pollution sources. While remediation of contaminated water is more successful with the efforts of many people, each individual can also make a significant contribution that should not be underestimated. Understanding the sources of pollution and making changes to reduce waste and pollution can have positive results. When contaminants do have to be removed, there are a number of processes that can be used, depending on the type and amount of pollution, and the size and location of the water body.
The Safe Drinking Water Foundation has educational programs that can supplement the information found in this fact sheet. Operation Water Drop looks at the chemical contaminants that are found in water; it is designed for a science class. Operation Water Flow looks at how water is used, where it comes from and how much it costs; it has lessons that are designed for Social Studies, Math, Biology, Chemistry and Science classes. Operation Water Spirit presents a First Nations perspective of water and the surrounding issues; it is designed for Native Studies or Social Studies classes. Operation Water Health looks at common health issues surrounding drinking water in Canada and around the world and is designed for a Health, Science and Social Studies collaboration. Operation Water Pollution focuses on how water pollution occurs and how it is cleaned up and has been designed for a Science and Social Studies collaboration. To access more information on these and other educational activities, as well as additional fact sheets, visit the Safe Drinking Water Foundation website at www.safewater.org.
Did you know that by using our Operation Water Pollution program in their classrooms, teachers educate students about what causes pollution, how it is cleaned up, and what they can do about the problem? Please help us to send more Operation Water Pollution kits to schools! Please chip in $5 or donate $20 or more and receive an Official Donation Receipt for Income Tax Purposes.
Resources:
Canadian Broadcasting Corporation. Februrary 2000. Environmentalists say pollution credits let companies pay to pollute. http://www.cbc.ca/news/canada/environmentalists-say-pollution-credits-let-companies-pay-to-pollute-1.207671
Environment Canada. November 2013. Full Text: The 2012 Great Lakes Water Quality Agreement. https://www.canada.ca/en/environment-climate-change/services/great-lakes-protection/2012-water-quality-agreement.html
Helmer, Richard, Hespanhol, Ivanildo & World Health Organization. (1997). Water pollution control : a guide to the use of water quality management principles. E & FN Spon. https://apps.who.int/iris/handle/10665/41967
Natural Ressources Defense Council. June 2006. Press Release : Smithsonian to Host Industry- Sponsored Exhibit on Tar Sands Oil Production: Environmental Consequences Are Ignored, Says NRDC. https://www.nrdc.org/media/2006/060607
Pelley, Janet. 2001. American Chemical Society : Ontario launches controversial smog trading program. https://pubs.acs.org/doi/pdf/10.1021/es022167t
Sawahel, Wagdy. June 2004. SciDevNet: Egyptian algae can clean up oil and soils.
http://www.scidev.net/global/news/egyptian-algae-can-clean-up-oil-and-soils.html
United States Environmental Protection Agency. 2021. Community Guide to Bioremediation. https://semspub.epa.gov/work/HQ/401583.pdf
United States Environmental Protection Agency. 2021. Community Guide to Granular Activated Carbon Treatment. https://semspub.epa.gov/work/HQ/401595.pdf
United States Environmental Protection Agency. 2021. Community Guide to In Situ Chemical Oxidation. https://semspub.epa.gov/work/HQ/401601.pdf
United Stated Environmental Protection Agency. 2021. Community Guide to Phytotechnologies. https://semspub.epa.gov/work/HQ/401615.pdf
United States Environmental Protection Agency. 2021. Community Guide to Pump and Treat. https://semspub.epa.gov/work/HQ/401617.pdf
United States Environmental Protection Agency. April 2007. Frequently Asked Questions About Water Quality Trading. https://www.epa.gov/npdes/frequently-asked-questions-about-water-quality-trading
World Health Organization. 2022. Water, sanitation and hygiene (WASH).
https://www.who.int/health-topics/water-sanitation-and-hygiene-wash